send link to app
cubeConsent app for iPhone and iPad
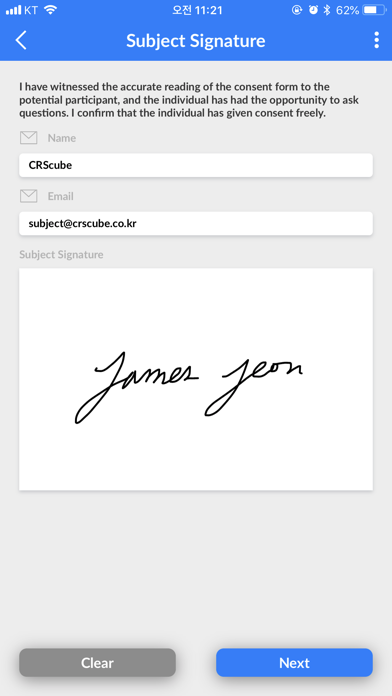
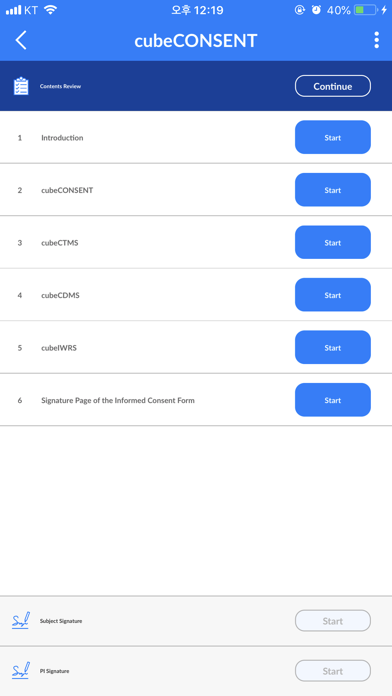
cubeCONSENT supports electronic Informed Consent From (ICF) solution.
It provides an optimal learning environment for future subjects and assures that the signing of the subjects consent is based on the patients accurate understanding on the test and participation in responsibility.
cubeCONSENT is interoperated with the cubeCDMS, capturing subjects informed consent information in real time.
cubeCONSENT Key features:
- Register a subject in cubeCDMS by signing the consent form
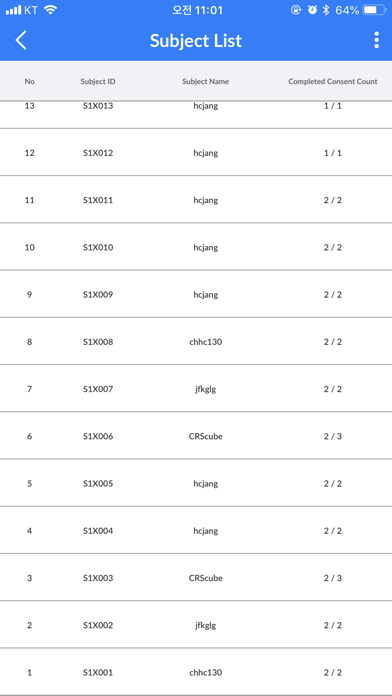
- Check the list of subjects registered in the study
- Sign the additional consent form for the subject